Recently, Professor Lu Ziyang and others from our institute published their latest research paper "Enhancing d/p-2 π * Orbitals Hybridization via Strain Engineering for Efficient Photoreduction CO2" in Angewandte Chemie International Edition, and it was selected for the Inside Cover. Jiangsu University is the first completion unit, with Zhou Guosheng and Liu Xinlin as co first authors, and Lu Ziyang and Liu Zhaoqing from Guangzhou University as co corresponding authors.
The utilization of CO2 resources helps to achieve energy conservation, emission reduction, and a green low-carbon economy. However, in the catalytic conversion process, the C=O bond has a high dissociation energy (about 750 kJ/mol), so the effective adsorption of inert CO2 on the active sites on the catalyst surface is a key step in the reaction. In addition, the activation of adsorbed CO2 into * COOH intermediates is the next key step in the photocatalytic CO2 reduction process, involving proton coupled electron transfer, which plays an important role in the entire photocatalytic CO2 reduction reaction and also involves the generation of various subsequent carbon containing products, especially for heterogeneous systems. Although strong adsorption (CO2 desorption temperature>400 ° C) can effectively activate CO2, stable chemical bonds and the required high reaction activation energy severely hinder the production of subsequent * COOH intermediates, greatly limiting the potential for catalytic conversion.
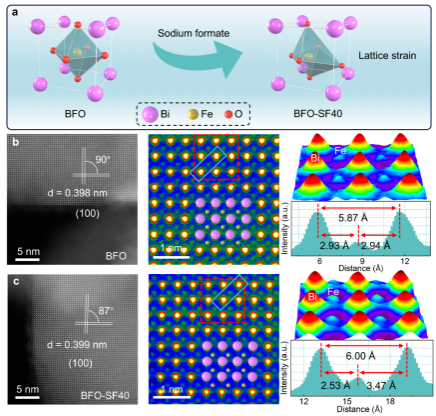
Therefore, rapidly converting strongly adsorbed CO2 into * COOH intermediates for efficient CO2 photoreduction is a major challenge. Utilizing solar energy to convert carbon dioxide into valuable chemical products is a promising strategy for addressing energy and environmental issues. However, strongly adsorbed CO2 significantly increases the activation energy of the reaction to hinder subsequent reactions. Here, this study proposes a BiFeO3 material with lattice strain, which synergistically regulates the d/p-2 π * orbital hybridization between metal sites and * CO2 and * COOH intermediates to achieve strong adsorption of CO2 and rapid conversion into key * COOH intermediates, accelerating the overall CO2 reduction kinetics. Theoretical calculations combining quasi in situ X-ray photoelectron spectroscopy and in situ Fourier transform infrared spectroscopy indicate that optimized Fe sites enhance the adsorption and activation of CO2. Under illumination conditions, internal electrons rapidly transfer to reaction sites and inject surface * CO2 and * COOH, promoting rapid activation and stability of * COOH.
This research achievement has received funding from projects such as the National Natural Science Foundation of China.

Article link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202411794